Water is an important part of our daily life and a basic need for survival. Among the many types of water available, boiling and distilled water are usually the most prominent. Although both have undergone a process of changing their composition, the question of their similarity and difference remains.
In this review, we explore the field of boiling and distilling, highlighting the effects each process has on water. When we embarked on this journey, our goal was to eliminate the question: “Is boiling water the same as distilled water?” The myth of the problem
What is distilled water?
Distillation is a laborious purification process that produces distilled water. This technique involves heating water to create steam, which is then cooled and condensed back into a liquid state. The magic of distillation lies in its ability to extract the purest water while removing impurities and minerals.
Distillation is just one method of purifying water; others include filtration, chlorination, and
reverse osmosis . The best water purification technology for homes that use public tap water is reverse osmosis.
Distillation removes impurities from water and makes it very pure. It can be used for a wide range of purposes such as medical applications, scientific research, and household appliances that require water with low mineral content. Due to its unmatched purity, distilled water meets exacting standards in industries where accuracy is critical.
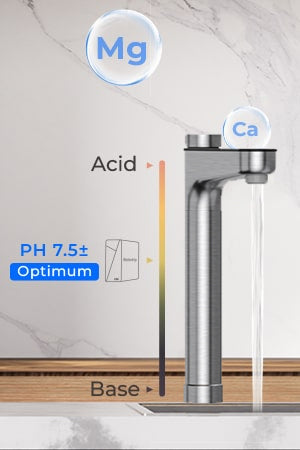
What is the pH of distilled water?
It is important to remember that the pH of water is not naturally 7, whether it is tap water, deionized water or distilled water. Carbon dioxide (CO2) dissolves in water when exposed to air and produces carbonic acid, so the actual pH of water is usually not 7.
What is boiling water?
The term "boiled tap water" describes a special type of water that is heated to a boil and then cooled naturally. A large number of bacteria and parasites found in raw water can cause disease in humans. It is recommended to drink boiled water because the boiling process burns any bacteria or parasites in the water.
Boiling water is an ancient method of filtering impurities and contaminants that humans have used for thousands of years. However, the effectiveness of boiled tap water as a purification technique is doubted. Whether you are in a remote place or at home, boiling water is a simple and effective way to purify it.
At what temperature does water boil?
The boiling process raises the temperature to 212 degrees Fahrenheit, which is crucial for killing harmful bacteria. In water heated to over 160 degrees Fahrenheit, most microorganisms take only 30 minutes to die.
The government will issue boil water advisories during natural disasters to ensure water safety. By neutralizing dangerous pathogens, this preventive measure protects public health by eliminating potential contaminants. Boiling is a simple and effective way to ensure that water is safe to drink and reduce health risks in unpleasant situations.
What is the difference between boiling and distilled water?
decontamination process
The simple process of heating water to the boiling point is called boiling. Heat treatment effectively eliminates dangerous microorganisms and ensures that the water is safe to drink. Although it addresses microbiological problems, it does not remove minerals and other contaminants from the water.
A more advanced purification method is distillation. Water is heated to steam and condenses to a liquid state after removing minerals and impurities. This methodical method guarantees the elimination of impurities and produces a very pure water that is obviously free of minerals and other contaminants.
Mineral composition
The minerals in tap water are naturally retained in boiling water. The water retains the same mineral content as spring water because no minerals are removed during boiling. Depending on the mineral content of the tap water, this can affect the taste and general composition of the water.
Distilled water, on the other hand, does not contain any minerals. The water goes through a distillation process to remove minerals and other impurities, leaving water with a much lower mineral content. The high purity of distilled water makes it suitable for use in situations where the absence of minerals is crucial, such as in scientific research or medicine.
effects on microorganisms
Boiling water is an effective method of removing dangerous microorganisms. Boiling breaks down the water and kills bacteria, viruses and other pathogens, making it safe for humans to drink. This method is especially useful when waterborne infections are a problem.
To remove microorganisms, distillation is equally effective. Contaminants, including microorganisms, are eliminated by turning water into steam and then into liquid. This guarantees that distilled water is free of biological contaminants, making it a reliable choice in applications where microbiological purity is critical.
taste and smell
The taste of contaminants found in source water may remain in boiled water. Minerals, chemicals and other substances present in the original water source can affect the taste of the water because boiling does not remove the flavor-related components.
A common characteristic of distilled water is the absence of taste and odor. The distillation process eliminates volatile compounds, minerals and impurities that affect taste and smell. Therefore, deionized water is generally odorless and tasteless and is a good choice when a clear, neutral taste is needed.
Application
Boiling water is a popular choice for cooking and drinking. The main benefit is that it removes harmful microorganisms, which means you can use it to cook food and drinks. A simple way to ensure the safety of everyday water is to boil it.
Special environments where high purity is crucial, such as laboratories and medical procedures, prefer distilled water. Distilled water is a reliable solvent in scientific research and medical equipment because it does not contain any minerals or impurities. In addition, it prevents the buildup of limescale in some household appliances such as steam irons that use mineral-free water.
How to Make Distilled Water?
Where to buy distilled water can be a problem for some people. Although it can be done at home, distilled water can also be bought from stores like supermarkets. Now let's look at how to make distilled water at home.
To make distilled water at home, you need to prepare the following supplies:
- kettle with lid
- A smaller bowl
- Ice
- Water
Fill your pot with half the water. Place an empty bowl in your pot. The bowl should not touch or sink to the bottom of the pot; instead, it should be smaller than a saucepan and float in the water. Turn the heat of the stove to medium heat and bring the water to a boil. After that, turn the lid upside down and cover with ice.
Condensation occurs when steam rises from boiling water and hits the ice-cold lid. Once the condensate falls into the bowl, you have distilled water. Continue distilling until you have the amount of water you need.
Is distilled water drinkable?
While it's technically safe to drink distilled water, using distilled water regularly may not be the best choice. The water is distilled to remove minerals, pollutants and impurities, leaving behind a very pure H2O. However, distilled water lacks the taste and health benefits that minerals like calcium and magnesium can provide.
It's usually safe to drink distilled water occasionally. But in the long run, it's probably not the best idea to rely on it as your primary source of hydration. The method of balancing water is usually a good idea, using a variety of sources to ensure you get the necessary minerals from food.